

This is because we’re adding more layers of electrons, smothering the positive protons at the centre. What about from up and down?Īs you go down a group (a column) in the Periodic Table, the atomic radius starts to increase. The overall effect is to pull the outer shell of electrons closer towards the centre of the atom. These additional protons add more positive charge to the nucleus, which means that the nucleus has a stronger “pull”, or attraction, on the negatively charged electrons (opposites attract). Why is this? Remember that as you go to the right across a row, you’re adding protons to the nucleus. This means that atomic size slowly decreases as you go from left to right. So, how does the Periodic Table give us a general trend for atomic radius? As you go from left to right across a period of elements (within the same row), you find the larger atoms on the left and the smaller atoms on the right. When atoms combine to form molecules, and those molecules go on to do their jobs, we need to know if an atom is very big and bulky or if it’s small and doesn’t take up much room. The atomic radius of iron metal is only 0.000000000126 meters! Knowing the size of an atom is important. Half of the length of that line is the atomic radius. You can draw a horizontal line between the centre of the two atoms, like this: Imagine placing two atoms side by side next to each other. Because the outer edge of an atom (the border) is a little “fuzzy” – the electrons aren’t really pinned down, and sort of surround the nucleus in a cloud – we calculate atomic radius in a different way than a normal circle. We can imagine atoms as circles, and just like circles, atoms have radii. If you half that distance, that’s the radius of the circle. If you draw a straight line through the centre of a circle, that’s called the diameter. You’ve probably learned from math class that a circle has a radius.
#Periodic table of elements with trends how to
You’re about to learn how to make four important predictions about atoms – atomic radius, ionisation energy, electron affinity, and electronegativity – just by judging how far “up or down” and “left to right” the element is in the Table. A vertical line of elements going up and down the table is called a column, or a group. Remember from your class that a horizontal line of elements going across the table is called a row, or a period. We’re going to give you some basic “left to right” and “up to down” rules. That’s what we’re going to be talking about today. Chemists could use these trends to make instant predictions about an element, without having to use any math at all.
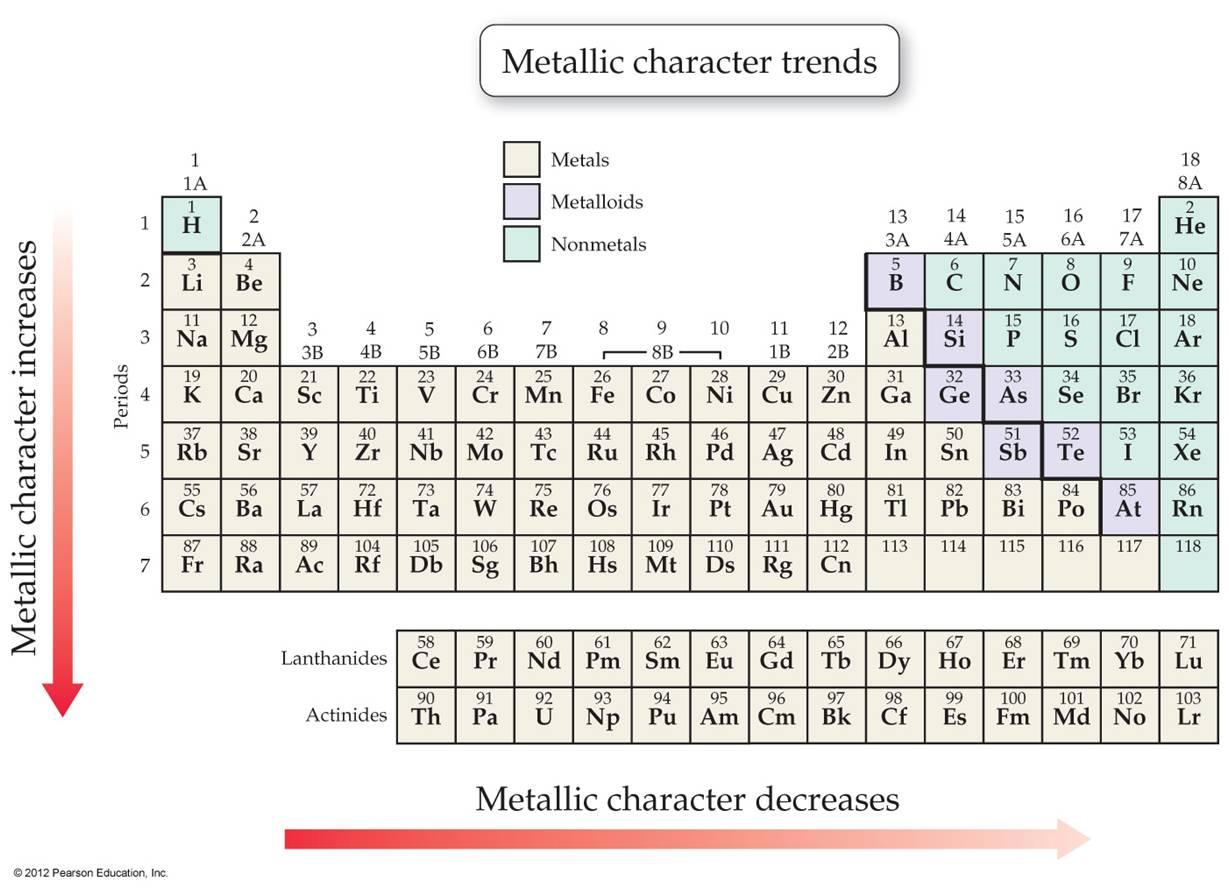
When some of the pioneers of chemistry – Dimitri Mendeleev, Antoine Lavosier, and others – put together the puzzle pieces of the different elements, they intentionally arranged the elements table into its current form so that trends would start to pop out. You don’t even have to pay attention to the numbers! All you have to do is locate an element on the Periodic Table, and the location alone is going to give you a lot of useful information. Don’t worry! With this guide you’re reading, you don’t have to worry about the atomic weights. While all of the information is really useful, it can sometimes be hard to keep everything straight. All the strange combination of letters, all the numbers – it can be very confusing. It might be hanging from a wall on a poster, or inside the back cover of your textbook. If you’re taking a chemistry class or even a general science class, chances are pretty good that you have a copy of the Periodic Table of Elements.


 0 kommentar(er)
0 kommentar(er)
